Coordinator – Dr. Stepan Tatikyan
Coordinator – Dr. Stepan Tatikyan
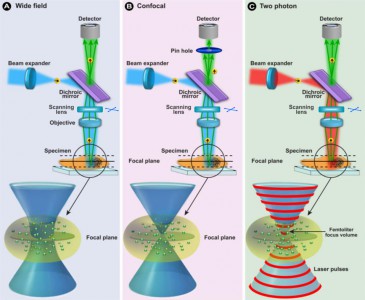
Our Two-Photon Fluorescence Laser Scanning Microscopy System (MOM Sutter instruments) is available for users from other institutions. For sample excitation Amplitude System infrared pulse laser is used.
Wavelength: 1030 nm
Energy: up to 25 nJ
Pulse length: 280 fs
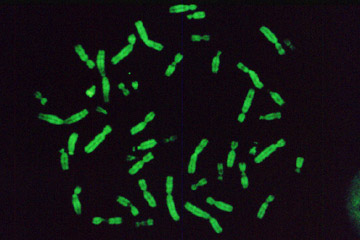
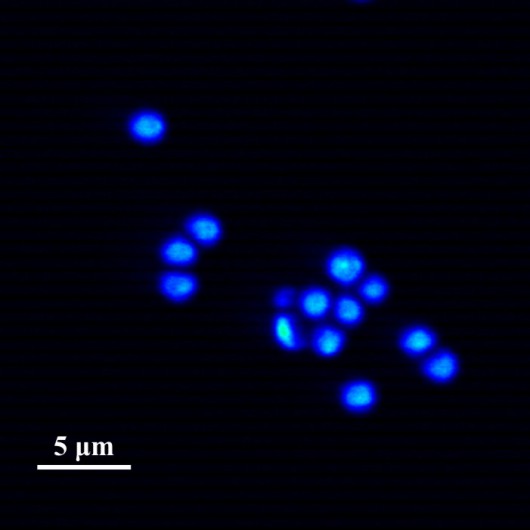 Test sample obtained by Sutter MOM microscope. Microcapsules with fluorescent dye.
Test sample obtained by Sutter MOM microscope. Microcapsules with fluorescent dye.
If you are interested in microscope usage please contact coordinator.
Application of 2 photon Fluorescence Microscopy
2 Photon Fluorescence Microscopy is widely used in many fields of:
- medicine
- biochemistry
- genetics
- histology
- virusology
- microbiology
- environmental sciences
- etc
Multiphoton Laser Fluorescence microscopy
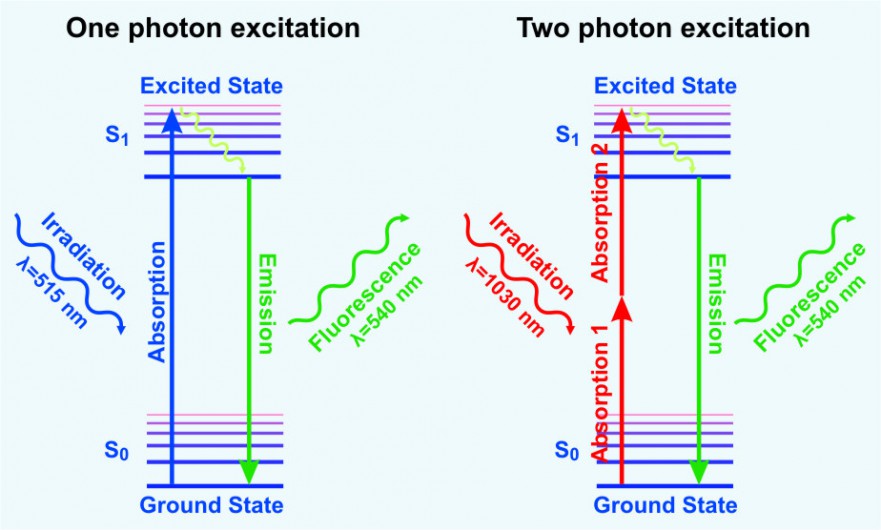
Multiphoton fluorescence microscopy is a powerful research tool that combines the advanced optical techniques of laser scanning microscopy with long wavelength multiphoton fluorescence excitation to capture high-resolution, three-dimensional images of specimens tagged with specific fluorophores.
The concept of two-photon excitation is based on the idea that two photons of comparably lower energy than needed for one photon excitation can also excite a fluorophore in one quantum event. Each photon carries approximately half of the energy necessary to excite the molecule. An excitation results in the subsequent emission of a fluorescence photon, typically at a higher energy than either of the two excitatory photons.
Three-photon excitation is a related non-linear optical absorption event that can occur in a manner similar to two-photon excitation. The difference is that three photons must interact simultaneously with the fluorophore to illicit a transition to the excited singlet state. A benefit of three-photon excitation is that successful absorption requires only a tenfold greater concentration of photons than two-photon absorption, making this technique attractive for some experiments.
The methodology is particularly useful to cell biologists who endeavor to study dynamic processes in living cells and tissues without inflicting significant, and often lethal damage to the specimen. Although classical widefield fluorescence microscopy can often provide submicron resolution of biochemical events in living systems, the technique is limited in sensitivity and spatial resolution by background noise caused by secondary fluorescence throughout areas situated above and below the focal plane.
Excitation in multiphoton microscopy occurs only at the focal point of a diffraction-limited microscope, providing the ability to optically section thick biological specimens in order to obtain three-dimensional resolution. Individual optical sections are acquired by raster scanning the specimen in the x-y plane, and a full three-dimensional image is composed by serially scanning the specimen at sequential z positions. Because the position of the focal point can be accurately determined and controlled, multiphoton fluorescence is useful for probing selected regions beneath the specimen surface.
Comparison of the Fluorescence Microscopy Techniques
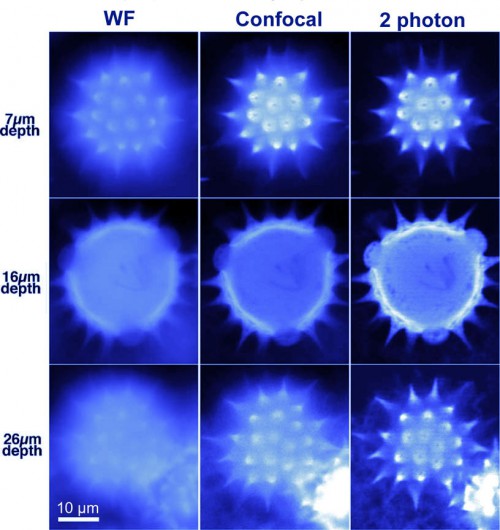 |
| Fluorescent pollen grain was imaged at three depths below its surface (7, 16, and 26 μm) by widefiled, confocal and 2 photon technique. Two-photon imaging improves image sharpness for deep optical sections by reducing scattering of excitation light and by eliminating fluorescence excitation outside the plane of focus. |
Advantages of 2 Photon Laser Scanning Microscopy (2P-LSM)
- 2P-LSM is more sensitive than Confocal Laser Scanning Microscopy (CLSM) because all the light generated to make an image is sent directly to the photon multiplier tube.
- This contrasts with CLSM where a pin hole is required to select the light from the focal plane. In CLSM there is considerable loss of signal in the optics required to direct the light to the pin hole.
- 2P-LSM gives a sharper image than CLSM because of the lack of extraneous light and improved geometry of detection.
- 2P-LSM uses solid-state lasers which are more stable than the gas lasers used in CLSM.
- The longer wavelength light used in 2P-LSM is more penetrating than the shorter wavelength used in CLSM (5 times deeper into biological material – up to 1 mm).
- The tissue above and below the plane of focus is merely subjected to infrared light and 2-photon excitation is restricted to a small focal volume.
- 2P-LSM microscopy separates excitation and emission light more effectively and has better signal/background ratio. A wider gap between excitation and emission makes it easier to reject excitation light, with minimal loss of emission photons.
- Due to reduced phototoxicity of IR beam two-photon microscopes are less damaging the sample than a single-photon confocal microscope.
Experiments
Institute of Molecular Biology
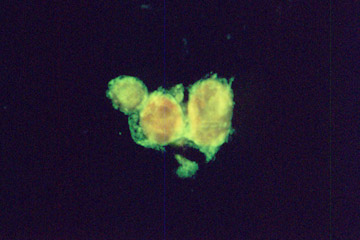
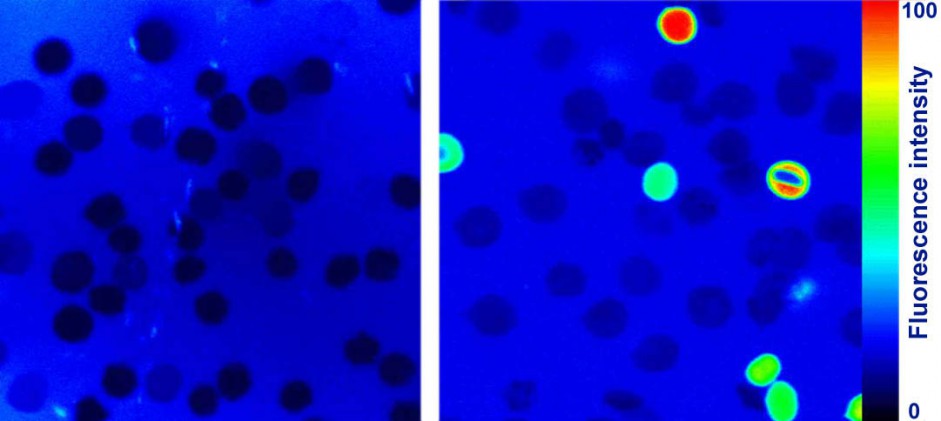
Investigation of red blood cells shock.
Native (intact) red blood cells do not possess fluorescence (left). After the shock caused by the hydrogen peroxide, damaged cells interact with the fluorescence marker (right). The fluorescence intensity is directly proportional to the degree of damage.
Institute of Biotechnology
Investigation of the joint fermentation of lactobacteria and yeast for obtaining biologically active compounds
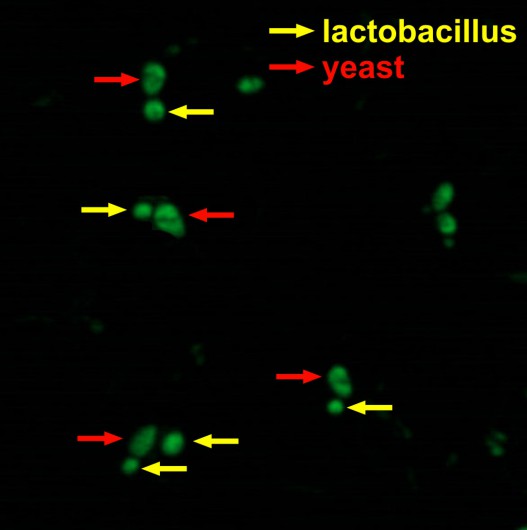 As the preliminary results show, lactobacteria and yeast interact with each other at the joint fermentation. communicate with each other.
As the preliminary results show, lactobacteria and yeast interact with each other at the joint fermentation. communicate with each other.
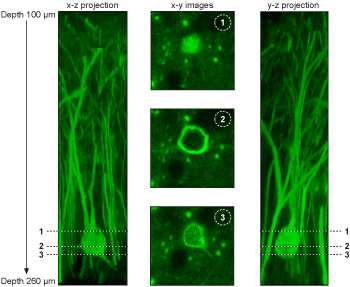
3D Imaging
Pollen grain 3D image. The x,y,z scanning results are reconstructed by ImageJ.